Origin and Spread | Maps | Identification | Biology | Ecology | Ecological Impacts | Economic Impacts | Control and Management | New York Distribution Map
Additional Common Names
Asiatic clam, Prosperity clam, Pygmy Clam, Golden clam, Good luck clam
Origin and Spread
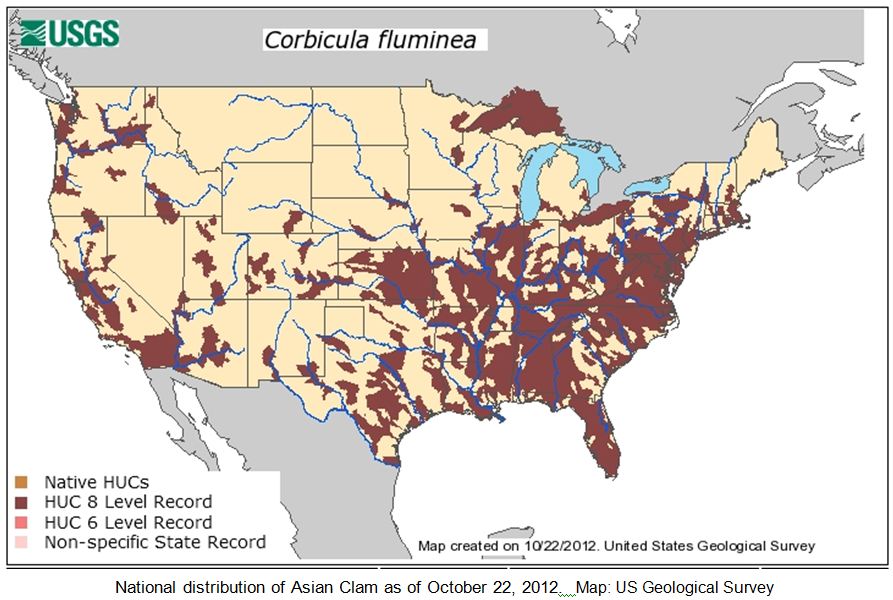
The Asian Clam Corbicula fluminea (Müller) is native to the fresh waters of eastern and southern Asia. It was likely introduced to the West Coast of North America around 1930, initially assumed to have been imported as a food source for the immigrating Chinese population (USACE ERDC 2007). Alternatively, it may have come in with the importation of the Giant Pacific oyster also from the Asia (Foster 2012). Live Asian clams were first detected in US waters in 1938 in the Columbia River, Washington; the species quickly spread across the continent and is currently found in 44 states. Corbicula was detected in the Ohio River in 1957 and continues to spread through drainages in the Midwest and Northeast. In New York State, the Asian clam has been collected in the running waters of central and western portions of the state, the Erie Canal from Lockport east to Clyde (shells only, colonization status unknown), Canandaigua Lake Outlet (shells only, colonization status unknown), Canandaigua, Keuka, Otisco, Owasco, and Seneca Lakes in the Finger Lakes, the Hudson River from Troy to Newburgh, the Wallkill River (colonization status unknown), the Champlain Canal near Fort Edwards, Lake George, and in Massapequa Lake and a number of other ponds and streams and the Massapequa Reservoir on Long Island.
The exact mechanism for secondary dispersal of Corbicula throughout North America is unknown, but likely involves human activity, including bait bucket introductions, accidental introductions associated with imported aquaculture species, and intentional introductions by people who buy them as a food item in markets (Foster 2012). Larval clams can attach to vegetation, floating debris for long distance dispersal. There is no peer-reviewed data demonstrating long distance overland travel by juveniles as a result of byssal attachment to trailered boats (McMahon 2012). Juvenile Corbicula are more likely to be carried in bilge and livewell water in boats and on vegetation attached to anchors and trailers or in sediments left on anchors (McMahon 2012).The only other significant dispersal agent is thought to be passive movement via water currents. There remains some question regarding transport by waterfowl. Based upon Foster (2012), birds are not considered to be significant distribution vectors. Sousa, et. al. (2008) cites a number of credible peer-reviewed sources indicating that transport on the feathers and feet of water birds is a secondary transport vector.
Identification
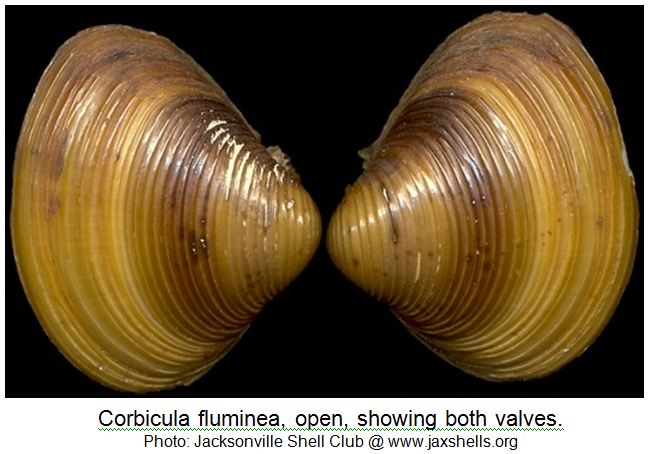
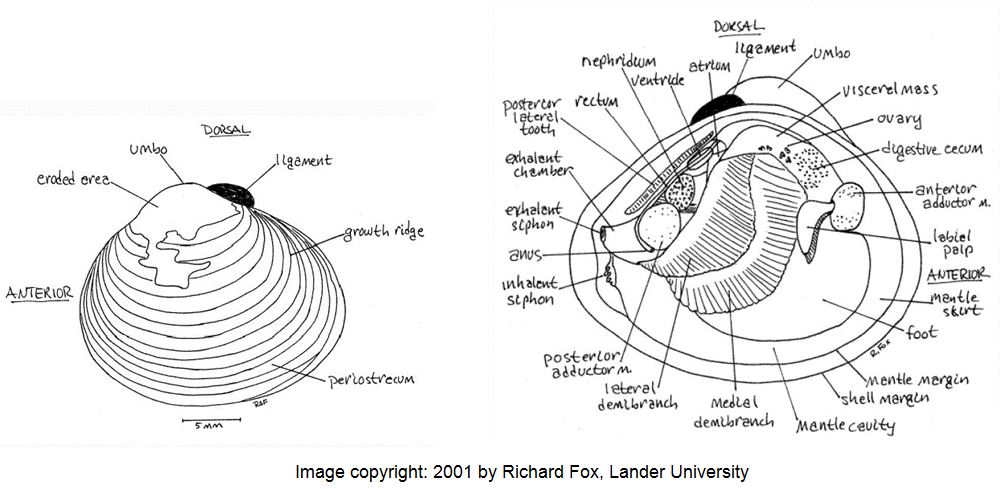
The species has a typical oval-triangular clam shape, with a dorsal “beak” or umbo at the peak of the shell. The outside of the shell (periostracum) is olive, or yellowish to black-brown in color, with 1-3 brown/purple colored radial bands (particularly in juveniles) and white erosion rings near the umbo. As the clam ages, the periostracum becomes darker in color. There are also distinctive, thick, concentric growth rings on the periostracum. The inside layer of the shell, the nacre (or “mother of pearl layer) is typically white-bluish white in color. Inside each shell half (or valve), there are also 1-2 pair of small, elongated and , finely serrated lateral “teeth” that extend on either side from the umbo part way down on the inside edge of each valve. Also, the interior of each valve, immediately under the umbo, there are 3 cardinal “teeth” (MacNeill 2012). The clam most closely resembles native sphaerid (fingernail) clams, however, sphaerid clams are smaller (6-14 mm), more oval in shape, cream colored, have fine growth rings, lack serrations on the lateral teeth and are found completely buried in the sediment. These relatives of Corbicula are also found in slow flowing waters with poorer water quality.
Corbicula fluminea External (left valve) and Internal (right valve) body features
Corbicula fluminea identication key
Biology
Corbicula burrows into the bottom sediments of streams and lakes and has the ability to feed from both the water column and the substrate. It uses its siphon to filter feed suspended particles (particularly phytoplankton) from the water and its fleshy foot appendage to pedal feed on detritus in the sediment. Corbicula can live in a variety of substrates, but prefers sand and gravel, over silt hard surfaces (McMahon 1999).
Corbicula is less tolerant than native mussels to environmental fluctuations. It is extremely sensitive to low oxygen conditions, and consequently its distribution is restricted to well‐oxygenated streams and lake shallows. In its native semi‐tropical/tropical habitat, the Asian clam is rarely exposed to temperature extremes. Its northern distribution in North America is thought to be limited by a 2°C lower lethal limit, and reproduction requires sustained water temperatures of 15°‐16°C. Asian clams have been known to find temperature refuges in cooler waters heated by power plant discharge. Upper lethal limit is believed to be 30°-35°C (Foster 2012, MacNeill 2012). Corbicula has been found to be resistant to desiccation and can survive periods of low water in damp sand or mud (USACE ERDC 2007).
Adult Corbicula are simultaneous hermaphrodites (both male and female) that are capable of both cross and self‐fertilization; thus, it takes only 1 individual to start a population. [Note: some literature indicates that Corbicula also exists as male and female sexes; this idea might be a result of observation of cross fertilization between hermaphrodites.] Adults can live 3‐4 years, and typically reproduce two times a year, although some populations have been observed reproducing more often under optimal situations. A single adult can produce 1000 – 100,000 juveniles per year. Egg fertilization is internal and the larval clams are brooded on the gill where they transform into juveniles in about 4-5 days (MacNeill 2012). Juveniles are tiny (0.25mm) and are capable of long distance dispersal via stream transport and water currents (using a mucous “balloon” or “parachute” {MacNeill 2012}), or hitchhiking on animals, floating objects, or vegetation (to which they can attach via a byssal thread). The juveniles transform into a pediveliger (shelled juvenile equipped with a foot) and settle to the bottom at about 0.25 mm in size. Pediveligers can crawl around along the bottom and seek firm substrates where they attach (at about 1.0 to 1.5 mm in size) using a temporary byssus which eventually disappears (MacNeill 2012). Juvenile clams can reach maturity in 3‐6 months or about 6-10 mm in size, and reach 10 to 30 mm in size during their first year depending on food availability and temperatures (MacNeill 2012).
Corbicula can rapidly grow into dense populations (> 2,000 per square meter), but are prone to rapid die‐offs with sudden changes in temperature (hot or cold) and low oxygen. However, their life history traits (i.e., quick maturity, high fecundity) enable rapid re‐colonization and population recovery, even after near extirpation. Additionally, these traits allow the Asian clam to successfully colonize habitats disturbed by human activity (e.g., channels and impoundments) that are unsuitable for native mussels.
Ecology
Like other bivalves, Corbicula is a filter-feeder on microscopic plants, animals (including bacteria) and in the water column or in the sediments. Among other freshwater bivalves it has the highest rates of filtration rates (up to 1.3 liters/hr/clam), food consumption and growth of any species (MacNeill 2012). It is also extremely efficient in channeling consumed food for growth and reproduction. Its ability to reproduce rapidly, coupled with low tolerance of cold temperatures, can produce wide swings in population sizes from year to year in northern water bodies. Both yellow and brown morphs are simultaneous hermaphrodites and brood their larvae in the inner demibranchs (Foster 2012). The life span is about one to seven years. No large-scale geographic features function as dispersal barriers.
Impacts
Ecological Impacts
Like other invasive mussels (e.g. zebra and quagga mussels), Corbicula is highly successful coupling the nutrient and energy flows that occur in the water column and bottom sediments. With a high filtering capacity and population density, Corbicula filters out phytoplankton and other particles suspended in the water that are also important food sources for other filter‐feeding organisms. Unlike zebra and quagga mussels, Corbicula also uses its pedal foot to feed on organic material and tiny organisms (microbes, protists, meiofauna) in the sediment (Hakenkamp et al. 2001). Whether Corbicula depletes these food resources to the extent that it negatively affects other organisms (particularly native unionid and sphaeriid mussels) remains an open question (Strayer 1999).
Corbicula can affect aquatic ecosystem processes in other ways. Bivalves, particularly when in dense populations, excrete significant amounts of inorganic nutrients, particularly nitrogen that, in turn, can stimulate the growth of algae and macrophytes (Lauritsen and Mozley 1989, Sousa et al. 2008). Additionally, Asian clam mass mortality events that occur in the summer followed by the release of nutrients via decomposition may also have negative effects on water quality. The shells of dead Asian clams can also provide a hard substrate on soft sediments, creating new habitat for other species that prefer hard substrates (e.g., zebra mussels).
It should be noted that to date, there are few studies on the ecological impacts of the Asian clam on native biota (McMahon 1999). In fact, most studies examine a water body after Corbicula invasion, with no comparable information on the biota or environmental conditions pre‐invasion.
Moreover, recent justifications for expensive and intensive management actions (e.g., Lake Tahoe Asian Clam Response) overstate evidence in the scientific literature. For example, in “Asian clam (Corbicula fluminea) of Lake Tahoe: Preliminary scientific findings in support of a management plan,” Wittman et al. (2008) state that:
“Asian clam is known to aggressively outcompete native invertebrate communities.”
Here the report authors incorrectly interpret and cite the findings of Karatayev et al. (2003). Karatayev and colleagues studied Corbicula in a Texas reservoir. Although the Asian clam dominated the total animal biomass of the reservoir sediments (up to 95%), it was not associated with declines in native biodiversity. In fact, it was found to co‐occur with an abundant population of native unionid mussels.
Studies claiming that the Asian clam has an impact on native bivalves (particularly unionids) are often anecdotal and only report the spatial distribution of bivalves after invasion (Strayer 1999). They assume that non‐overlapping distributions of Corbicula and native mussels indicate that Corbicula have outcompeted the native species. As Strayer (1999) points out, this is just one possible explanation. Corbicula could also prefer different habitat than native mussels (e.g. sandy vs. silt/gravel). Native mussels have long experienced declines due to human‐induced changes to habitat (pollution, land use change, channelization), and it is difficult to tease apart these changes versus direct impacts of invading Corbicula that also happen to do well in disturbed habitats. Thus, while it is quite possible that in some cases Corbicula has a direct, negative impact on native biota, more studies that monitor changes in mussel populations over time and that directly evaluate competitive interactions are warranted.
Economic Impacts
Asian clams can colonize the intake pipes of water treatment systems and power stations. Unlike zebra mussels, Corbicula do not attach to the hard substrate. Rather, juvenile clams pass through filter screens/strainers and settle on the floors of intake pipes where low flow allows silt and sand to settle. The clams reproduce in situ and continue to accumulate in pipes and are transported deeper into the system. Corbicula fouls the pipes, blocking structures with shells, altering flow, and increasing sedimentation rates (McMahon 1999).
Asian clam shells that accumulate in beach or swimming areas will also impede recreation and tourism.
Control and Management
Little to no research regarding control practices have been published in the peer‐reviewed scientific literature. The Lake Tahoe team has submitted a manuscript for publication to the journal Biological Invasions on the use of bottom barriers to control Corbicula and it remains in the peer review process. Additionally, the New York Invasive Species Research Institute had difficulty locating research-based Best Management Practices for Corbicula.
To date, given the life history traits of Corbicula that make it a successful invader and the widespread distribution of the species in North America, eradication is typically not a viable option. As with many aquatic invaders, emphasis ought to shift from eradication to containment and spread prevention.
New York Distribution Map
This map shows confirmed observations (green points) submitted to the NYS Invasive Species Database. Absence of data does not necessarily mean absence of the species at that site, but that it has not been reported there. For more information, please visit iMapInvasives.
Acknowledgment
New York Invasive Species Information wishes to acknowledge the work of Dr. Holly Menninger, former director of the New York Invasive Species Research Institute. Dr. Menninger’s literature review formed the basis for this species profile and provided much of the text published above.





.JPG)